Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Composites
to Ceramic Matrix Composites
Interphases
Dr. Dmitri Kopeliovich
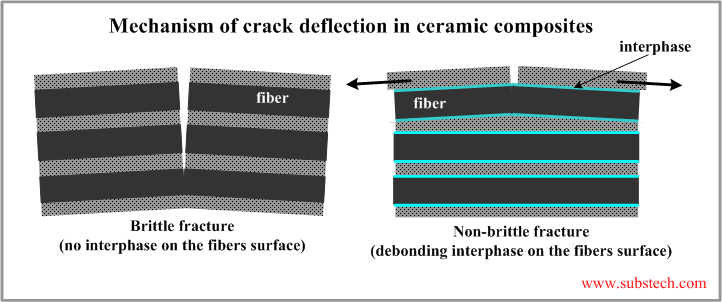
Interaction between the matrix and the reinforcing fibers provides higher toughness of a ceramic composite as compared to the matrix material in the monolithic state.
Such effect is a result of cracks deflection at the matrix-fiber interface. When a crack propagating through the matrix reaches a fiber, the relatively weak bonding (debonding) between the matrix and the fiber at their interface allows their relative sliding, which prevents the fiber fracture. The fiber bridges the cracked matrix.
The effect of the crack deflection mechanism is determined by the matrix-fiber bonding strength. If it is too great the fibers are not capable to slide in the matrix therefore the crack passes through the fibers breaking them. The fracture of the composite is brittle like in the monolithic Ceramics.
In most infiltration processes of fabrication of ceramic composites strong bonds between the matrix and the fibers form due to chemical interaction between the materials or due to their diffusion into each other. Debonding may be achieved if the fibers and the matrix are separated from each other with a a layer of an interphase preventing their interaction.
Additional interphase layers (for example a film of silicon carbide of the thickness 0.00002-0.0002”/0.5-5 μm) provide protection of the fibers from either environmental attacks (e.g. oxidation) or aggressive action of the infiltrated material (e.g. liquid silicon).
In order to provide weak bonding the interphase material should have a low shear strength. The materials with low shear strength have a layered crystalline structure composed of weak bonded layers allowing easy slippage between them (similar to Graphite): pyrolytic carbon (C) and hexagonal boron nitride (BN).
The structure of pyrolytic carbon is composed of graphene planes bonded to each other by weak Van der Waals forces. The structure of hexagonal boron nitride is also layered. The atoms of boron and nitrogen are strongly bonded within a layer however the bonding between the neigboring layers is weak.
An interphase film of 0.000004-0.00004” (0.1-1 μm) thickness is deposited prior to the infiltration of the matrix. Thicker interphase provides weaker matrix-fiber bonding. The method of chemical vapor infiltration (CVI) is commonly used for deposition of the interphases.
The interphases from pyrolytic carbon withstand high temperatures in non-oxidizing environments, however in air they oxidize and their maximum operation temperature is 932°F (500°C).
High purity hexagonal boron nitride may survive in a dry oxidizing atmosphere up to 2192°F (1200°C).
to top
Related internal links


