Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Polymers
Polymer structure
Dr. Dmitri Kopeliovich
Polymer is a substance (natural or synthetic), molecules of which consist of numerous small repeated chemical units (monomers) linked to each other in a regular pattern.
Polymers usually combine crystalline and amorphous structures (semi-crystalline).
Degree of polymerization is an average number of monomers (mers) in a polymer molecule.
Polymer molecules may combine up to million of monomers (mers) forming a one-dimensional structure (chain), two-dimensional structure (planar molecules) or three-dimensional structure.
One-dimensional structure is common for organic polymers.
Organic polymer is a polymer compound built of hydrocarbon base monomer units.
Besides carbon and Hydrogen the following atoms may be incorporated in polymer molecules: Oxygen, Nitrogen, chorine, fluorine, silicon, phosphorous, and sulfur.
Atoms of a polymer molecule are held by covalent bonding.
Neighboring chains may form secondary bonds between them (cross-links) which are less strong than covalent bonding between the atoms within the molecules.
Cross-links provide elasticity to the polymer, preventing sliding of the neighboring chains when the material is stretched.
Branched polymer consists of molecules having side chains (branches) attached to the main chain.
Copolymer is a polymer molecule of which contains more than one kind of monomers.
Nylon is a common copolymer. Its molecules consist of two alternating monomers: diacid and diamine.
Graft copolymer is a kind of branch polymer, side chains of which are made of monomers differing from the monomer of the main chain.
Block copolymer is a polymer molecules of which built from alternating polymeric blocks of two or more different polymers.
Structure parameters affecting polymer properties:
- Increase of the chain length.
Effect: increase of tensile strength and Modulus of Elasticity (stiffness).
- Increase of number and length of side chains.
Effect: increase of tensile strength and stiffness.
- Introduction of large monomers in molecules.
Effect: increase of stiffness.
- Increase of number and strength of cross-links.
Effect: increase of tensile strength and stiffness.
- Orientation of the molecules as a result of deformation during manufacturing.
Effect: anisotropy of the material properties (properties along the deformation differ from those in other directions).
Every polymer is characterized by a temperature below of which mobility of its molecules sharply decreases and the material becomes brittle and glassy.
This temperature is called Glass Transition Temperature.
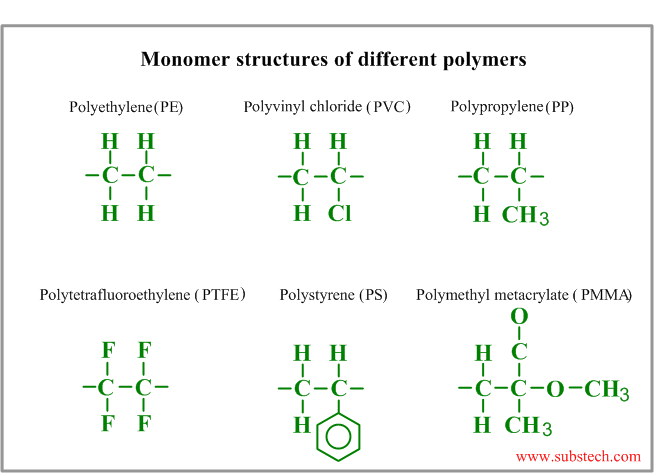
Monomer molecular structures of different polymers are presented in the picture:
Related internal links
to Polymers