to Metals
to Aluminum alloys
Degassing treatment of molten aluminum alloys
Dr. Dmitri Kopeliovich
Degassing of molten Aluminum alloys is a foundry operation aimed to remove Hydrogen dissolved in the melt.
Hydrogen in aluminum
Liquid aluminum actively dissolves hydrogen, which forms as a result of chemical reaction with water vapor:
2Al + 3H2O = Al2O3 + 6H
Solubility of gaseous hydrogen in liquid aluminum at its melting point (1220.7°F/660.4°C) is 0.61 in3/lb (2.2 cm3 per 100 g).
Solubility of gaseous hydrogen falls sharply when aluminum solidifies: solid aluminum at melting point contains only 0.014 in3/lb (0.05 cm3 per 100 g).
Therefore aluminum alloys release excessive amount of hydrogen during Solidification. This results in porosity defects distributed throughout the solid metal. Size of the hydrogen pores and their quantity is determined by the initial content of hydrogen, the alloy composition and the solidification conditions.
Sources of hydrogen in molten aluminum:
- atmosphere humidity;
- wet metallic charge;
- wet furnace lining (crucible, transfer ladles);
- wet foundry instruments;
- wet fluxes and other consumables;
- furnace fuel combustion products containing hydrogen.
Methods of hydrogen content estimation:
- Slow solidification. In this method a small portion of liquid aluminum (about 2 in3/33 cm3) is poured into a cavity in a heated refractory brick. The alloy slowly solidifies and the released hydrogen is concentrated in the upper part of the casting in form of frozen bubbles. Quantity of the hydrogen bubbles at the sample surface is determined by the hydrogen concentration.
- Vacuum method. This quantitative method uses solidification of a sample portion of the aluminum alloy in a small crucible at low pressure. Hydrogen dissolved in the alloy starts to form a gaseous phase (a bubble) at a certain pressure. When the first bubble is formed both the pressure and the temperature are measured. These parameters are used for determination of the hydrogen content by means of numeric diagrams.
Degassing by fluxes
Fluxes composed of chlorine and fluorine containing salts are used for degassing molten aluminum alloys. Degassing fluxes are commonly shaped in form of tablets.
Degassing operation starts when a flux tablet is plunged by a clean preheated perforated bell to the furnace bottom. The flux components react with aluminum forming gaseous compounds (aluminum chloride, aluminum fluoride). The gas is bubbling and rising through the melt. Partial pressure of hydrogen in the formed bubbles is very low therefore it diffuses from the molten aluminum into the bubbles. The bubbles escape from the melt and the gas is then removed by the exhausting system.
The process continues until bubbling ceases.
to top
Rotary degasser
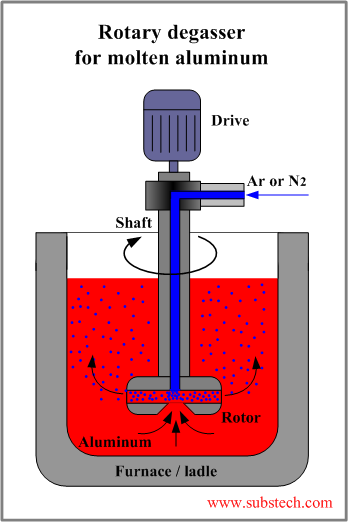 In the rotary degassing method an inert or chemically inactive gas (Argon, Nitrogen) is purged through a rotating shaft and rotor.
In the rotary degassing method an inert or chemically inactive gas (Argon, Nitrogen) is purged through a rotating shaft and rotor.
Energy of the rotating shaft causes formation of a large number of fine bubbles providing very high surface area-to volume ratio.
Large surface area promotes fast and effective diffusion of hydrogen into the gas bubbles resulting in equalizing activity of hydrogen in liquid and gaseous phases.
Rotary degasser allows achieve more complete hydrogen removal as compared to the flux degassing.
Additionally rotary degasser does not use harmful chlorine and fluorine containing salts.
Rotary degasser may also combine the functions of degassing and flux introduction.
In this case the inert gas serves as carrier for granulated flux. The method is called flux injection.
Advantages of flux injection:
- high effectiveness of flux action due to better mixing with the melt;
- short flux treatment time;
- controllable flux introduction;
- more environmentally friendly method of fluxing.