Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Steel making
Fabrication of large steel ingots
Dr. Dmitri Kopeliovich
Large steel ingots are required for manufacturing electric power plant turbine shafts, generator rotor shafts, nuclear pressure vessels, chemical pressure vessels, ship parts and other heavy machinery parts.
Metalforming technology used for final shaping of large ingots is Forging.
The largest ingot (570 metric tons) was produced in 1980 by “Kawasaki Steel” (Japan).
Solidification of a large mass of steel is characterized by significant development of micro and macro-defects of the ingot structure:
The structure defects decrease the reliability of the part manufactured from the ingot. Since such parts work in equipment, failure of which is potentially catastrophic (nuclear equipment, electric power plants, chemical equipment, large scale machinery), Technology of large ingots fabrication should provide minimum degree of steel structure defects.
Non-metallic inclusions
Non-metallic inclusions in steel are chemical compounds of metals (Fe, Mn, Al, Si, Ca) with non-metals (O, S, C, H, N). Non-metallic inclusions form separate phases. The non-metallic phases containing more than one compound (eg. different oxides, oxide+sulfide) are called complex non-metallic inclusions (spinels, oxysulfides, carbonitrides).
Despite small content of non-metallic inclusions in steel (0.01-0.02%) they exert significant effect on the steel properties such as:
The following parameters of non-metallic inclusions influence on the properties of parts made of large steel ingots:
-
- Oxides
- Sulfides
- Oxysulfides
- Carbides
- Nitrides
- Carbonitrides
- Phosphides
Size of non-metallic inclusions is determined by the processes of nucleation, growth and coalescence/agglomeration. High surface energy causes the nucleation at higher supersaturation of the solutes (oxygen, sulfur, nitrogen, aluminum, silicon, titanium, vanadium, etc.) and favors coalescence and agglomeration of the inclusions.
-
- Globular inclusion form in liquid state at low concentration of aluminum. Globular shape inclusions exert moderate influence on the steel properties therefore globular morphology is preferable.
- Platelet shaped inclusions form at the grain boundaries as a result of eutectic transformation during solidification. Platelet shaped inclusion exert adverse effect on the steel properties and therefore this morphology is undesirable.
- Dendrite shaped inclusions form at high concentration of aluminum. Their shape is characterized by sharp edges, which may cause concentration of stresses during the ingot forging and decrease of ductility, toughness and fatigue strength of the steel part fabricated from the ingot.
- Polyhedral inclusions are result of modification of the dendrite shaped inclusions by addition of strong deoxidizers and rare earth (Ce,La) or alkaline earth (Ca, Mg) elements. The effect of polyhedral inclusions on the steel properties is less than that of the dendrite shaped inclusions.
Homogeneous distribution of non-metallic inclusions is most desirable. Clusters of inclusions are unfavorable since they may result in local drop of mechanical properties such as toughness and fatigue strength.
- Physical and mechanical properties (hardness, ductility, melting point)
Microscopic hard inclusions (carbides, nitrides) strengthen the metal however larger hard inclusions may cause drop of the steel ductility without increase of the strength and hardness. Ductile and brittle inclusions behave different during plastic deformation (steel Forging). Ductile inclusions elongate in the direction of deformation. Brittle inclusions break to fragments and form chains.
Macrosegregation
The following factors favors macrosegregation in large steel ingots:
- Large absolute amount of solutes (sulfur, phosphorous, carbon). Steel is being enriched with the solutes rejected by the moving solidification front therefore at the final solidification stage the residual liquid contain large solute content.
- Low cooling rate of the metal in the central ingot zone. Low cooling rate results in larger spaces between the dendrite arms filled with the liquid metal enriched with the solutes. Partition of solutes between the dendrite arms and interdendritic liquid is called Microsegregation, which is the main cause of macrosegregation. Additionally solidification conditions at low cooling rate are closer to equilibrium resulting in decrease of Equilibrium Partition Coefficient and higher microsegregation.
- Large distances, which liquid metal and separate dendrite crystals and their fragments may be transferred. Transfer of liquid and solid phases in a solidifying ingot is the result of solidification shrinkage, buoyancy forces acting on the liquid metal, sedimentation of solid crystals homogeneously nucleated in liquid phase and fragments of broken or melted off dendrites.
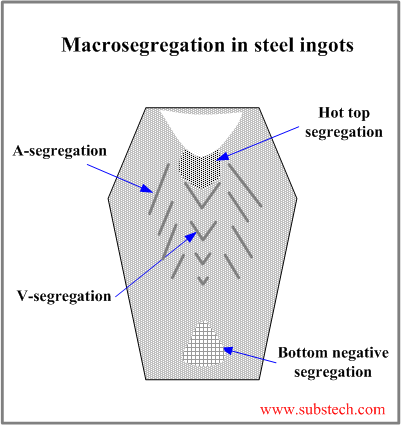 Development of macrosegregation zones in a steel ingot and their locations are associated with the ingot grain structure.
Development of macrosegregation zones in a steel ingot and their locations are associated with the ingot grain structure.
- Bottom negative segregation
Bottom negative segregation is a result of low solute concentration in the crystals formed in the early stage of solidification and comprising bottom cone. The bottom cone is a mixture of small equiaxed garins grown as a result of the contact with a bottom of a cold metallic mold and crystals and crystals fragments, which sedimintate from other ingot zones.
- V-segregation
The central zone of ingot is enriched with solute rejected by the solidification front progressing from the mold wall to its center. The central zone consists of large equiaxed grains, which settle down to the V-shaped solidification front. The residual liquid surrounding the large equiaxed grains is solute-rich and it forms V-segregates when solidifies.
- A-segregation
A-segregates (freckles) form in the Zone of columnar grains at the regions with structure characterized by the transition from the columnar grains to large equiaxed grains. A-segrgates present channels enriched by sulfur, carbon, phosphorus and other impurities.
- Hot top segregation
Hot top segregation zone is located in the top central ingot region below the shrinkage cavity. Hot top segregation is formed at the final solidification stage from the residual liquid enriched by the solutes as a result of microsegregation (rejection by solidifying dendrites) followed by penetration of the liquid through the dendrite skeleton.
Factors allowing to diminish macrosegregation in large steel ingots:
- Lowering contents of impurities. Steel for large ingots is treated in the melting furnace and in Ladle refining stands in order to remove undesirable impurities such as sulfur (desulfurization]]), phosphorous (dephosphorization), hydrogen (degassing). Steel for large steel ingots commonly contains not more than 0.005% of sulfur, 0.005 of phosphorous and up to 2 ppm of hydrogen.
- Alloying of steel by alkaline (Ca, Mg) or rare earth (Ce,La) elements in amount of about 2*[S].
- Low concentration of silicon in steel (about 0.1%).
- Using steel grades with lower carbon content.
- Modification of the ingot dimensions. If low level of A-segregation is required the ratio of the ingot height to its diameter should be as low as possible (about 1.0-1.2).
- Decrease of the pouring temperature. Lower pouring temperature results in increase of the cooling rate, which is favorable for depressing macrosegregation.
Hydrogen in steel
Sources of hydrogen in liquid steel:
- Damp scrap;
- Fluxes and alloying additives;
- Furnace and ladle refractories;
- Atmospheric humidity;
- Fuel (if used).
Hydrogen is easily dissolved in liquid steel in dissociated (atomic) state. Solubility of hydrogen in steel drops sharply during solidification resulting in formation of gaseous hydrogen form H2. In solid steel hydrogen is dissolved in form of interstitial solution.
Carbon, nickel, chromium (up to 10%), vanadium, titanium, zirconium, columbium, tantalum increase the solubility of hydrogen in solid steel.
Silicon, aluminum, tungsten, chromium (10% and higher) decrease the solubility of hydrogen in solid steel.
Solubility of hydrogen in austenite is much higher than in ferrite.
Both gaseous and dissolved forms of hydrogen exert adverse effect on mechanical properties of steels:
- Hydrogen flakes
Solubility of hydrogen decrease during solidification and cooling down of steel ingot. Hydrogen atoms possessing high mobility are collected at internal voids such as non-metallic inclusions (sulfides, oxides) and their clusters, shrinkage pores, cracks caused by internal stresses.
Hydrogen atoms collected at internal voids combine and form gaseous hydrogen H2, which may cause formation of cracks (flakes) when the gas pressure exceeds the steel strength.
Hydrogen flakes is particularly dangerous for parts fabricated from large ingots. Vacuum ladle degassing methods allow to decrease the content of hydrogen to 2 ppm, which does not cause flaking formation.
- Hydrogen embrittlement
Hydrogen in dissolved form also decreases steel properties such as ductility, Fracture Toughness and fatigue strength.
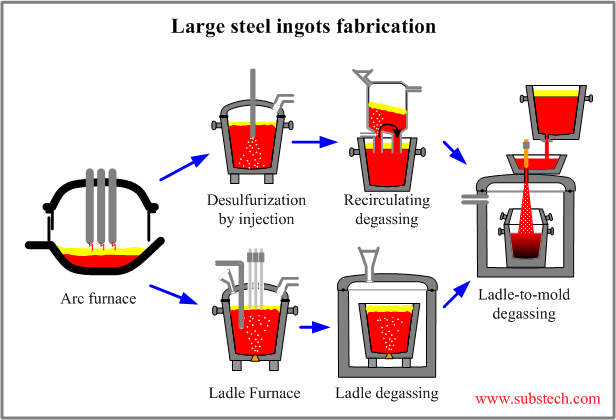
Technology of large ingots fabrication
The following tasks are accomplished by the technology of large ingots fabrication:
- Dephosphorization (to about 0.005%) in a steel making furnace (melting furnace).
- Desulfurization of steel (to about 0.005%) in the melting furnace and then by a ladle refining desulfurization method (eg. Ladle desulfurization by injection of active agents, Ladle Furnace (LF)).
- Deoxidation of steel by metallic deoxidizers to the required concentrations of aluminum, silicon and other deoxidizing elements.
- Removal of non-metallic inclusions from the liquid steel in both melting furnace and Ladle refining stand.
- Vacuum treatment of steel in ladle by one of the degassing methods: Recirculation Degassing (RH), Ladle Degassing (VD).
- *Achievement of the required temperature of steel before pouring to the mold by using Ladle Furnace (LF).
- Final vacuum treatment of the steel in the Ladle-to-mold degassing operation.
- Providing non-intermittent supply of liquid steel to the pouring-to-mold operation.
The possible technological schemes of large steel ingots fabrication are presented in the figure:
to top
Related internal links
to Metals
to Steel making


