to Metals
to Corrosion and oxidation
Pitting corrosion
Dr. Dmitri Kopeliovich
Pitting corrosion is an electrochemical oxidation-reduction (redox) process, which occurs within localized holes (cells) on the surface of metals coated with a passive film.
Pitting corrosion is considered much more dangerous than uniform corrosion since its rate is 10-100 times higher.
Pitting corrosion is highly accelerated if chloride, sulphate or bromide ions are present in the electrolyte solution.
Stainless steels and other metals forming a passive oxide layers on their surfaces (Aluminum alloys, Copper alloys, chromium) in electrolytes and atmosphere are sensitive to pitting corrosion.
Mechanism of pitting corrosion is similar to that of Crevice corrosion: dissolution of the passivating film and gradual acidification of the electrolyte caused by its insufficient aeration (Oxygen penetration).
Stages of pitting corrosion:
- Pit initiation. An initial pit may form on the surface covered by a passive oxide film as a result of the following:
- Mechanical damage of the passive film caused by scratches. Anodic reaction starts on the metal surface exposed to the electrolyte. The passivated surrounding surface act as the cathode.
- Particles of a second phase (non-metallic inclusions, intermetallic inclusions, metallic particles, Microsegregation) emerging on the metal surface. These particles precipitating along the grains boundaries may function as local anodes causing localized galvanic corrosion and formation of initial pits.
- Localized stresses in form of dislocations emerging on the surface may become anodes and initiate pits.
- Non-homogeneous environment may dissolve the passive film at certain locations where initial pits form.
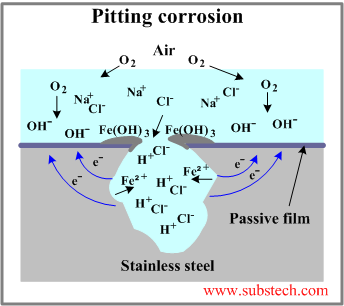
- Pit growth. In presence of chloride ions pits are growing by autocatalytic mechanism. Pitting corrosion of a stainless steel is illustrated in the figure.
Anodic reactions inside the pit:
Fe = Fe2+ + 2e- (dissolution of iron)
The electrons given up by the anode flow to the cathode (passivated surface) where they are discharged in the cathodic reaction:
1/2O2 + H2O + 2e- = 2(OH-)
As a result of these reactions the electrolyte enclosed in the pit gains positive electrical charge in contrast to the electrolyte surrounding the pit, which becomes negatively charged.
The positively charged pit attracts negative ions of chlorine Cl- increasing acidity of the electrolyte according to the reaction:
FeCl2 + 2H2O = Fe(OH)2 + 2HCl
PH of the electrolyte inside the pit decreases (acidity increases) from 6 to 2-3, which causes further acceleration of corrosion process.
Large ratio between the anode and cathode areas favors increase of the corrosion rate.
Corrosion products (Fe(OH)3) form around the pit resulting in further separation of its electrolyte.
Means of pitting corrosion control:
- Selection of appropriate material;
- Providing stirring of the electrolyte;
- Control of the electrolyte composition (PH, chloride ions);