Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Corrosion and oxidation
Galvanic corrosion
Dr. Dmitri Kopeliovich
Galvanic corrosion is an electrochemical oxidation-reduction (redox) process, which occurs when two dissimilar metals or alloys are brought into electrical contact and immersed into an electrolyte solution.
In practice the electrolytes are aqueous solutions of salts, acids and bases.
Electrochemistry of galvanic corrosion
The two metals immersed into an electrolyte forms a galvanic cell, in which the metal having lower value of electrode potential (higher position in the table of Electrochemical series) will oxidize (anodic reaction) and the metal having higher value of electrode potential (more noble) will provide cathodic reaction (reduction) on its surface.
The algebraic difference between the electrode potentials of the two metals determines the driving force of galvanic corrosion.
Two examples of galvanic corrosion are presented in the figure below.
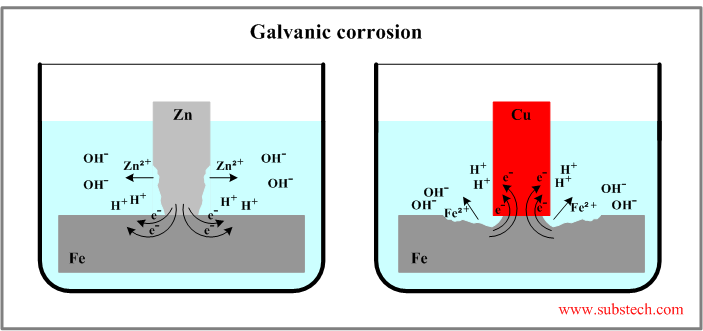
System iron-zinc
The Standard electrode potentials of the metals: E0Zn = -0.763 V, E0Fe = -0.44 V. The difference: E0Fe - E0Zn = 0.323 V.
The potential of Zn is lower therefore it dissolves in electrolyte according to anodic reaction: Zn = Zn2+ + 2e- (corrosion of zinc).
The electrons given up by the anode flow to the cathode (iron) where they are discharged in the cathodic reaction: H+ + e- = H.
System iron-copper
The standard electrode potentials: E0Fe = -0.44 V, E0Cu = +0.337 V. The difference: E0Cu - E0Fe = 0.777 V.
Potential of iron is lower therefore it reacts anodically (dissolves) in this system: Fe = Fe2+ + 2e- (corrosion of iron).
Cathodic reaction of the electrons with hydrogen ions occurs on the copper surface.
The cathode may be polarized by the hydrogen atoms producing a film covering the cathode surface. The film affects corrosion kinetic: it slows down the reaction between the electrons and hydrogen ions dissolved in the electrolyte. Slowing cathodic reaction causes slowing anodic reaction, which are linked to each other.
In the electrolytes with high concentration of hydrogen ions (acidic solutions) the hydrogen atoms adsorbed on the cathode surface form hydrogen gas escaping from the cathode and promoting corrosion: 2H = H2.
Common aqueous solutions are aerated (contain dissolved oxygen) therefore hydrogen atoms formed on the cathode surface react with oxygen: 1/2O2 + 2H = H2O.
Kinetic of the process in this case is determined by the diffusion of oxygen to the cathode surface.
to top
Factors of galvanic corrosion
The following factors determine thermodynamic and kinetic conditions of galvanic corrosion:
- Difference between the electrode potential of the two metals. The greater the difference the higher the driving electric force of corrosion.
- Contact resistance at the boundary between the two metals. High contact resistance limits the electrons transfer through the boundary and decrease the corrosion rate.
- Electric resistance of electrolyte solution. Dilute solutions having high electric resistance provide low corrosion rate.
- Anode-to-cathode areas ratio. Large anode connected to a small cathode result in low corrosion rate.
- Presence of passive film.
- Electrolyte solution properties (PH, oxygen content, temperature, flow rate).
Passivation
Passivation is a formation of a thin film of oxidation products preventing further corrosion of the metal.
The oxide passivation film my form either on exposure to air or in electrolyte with presence of oxygen.
Conditions (electrode potential vs. PH)of formation of passive film are determined by the Pourbaix diagrams.
Low carbon steel does not corrode in concentrated nitric acid due to protection effect of passive film however dilute nitric acid does not form stable passive film and therefore easily dissolves steel.
Passive film is formed on the surface of aluminum, chromium, silicone and titanium in air, water and dilute acids.
Good corrosion and oxidation resistance of Stainless steels is also a result of formation of chromium reach oxide passive layer. The passivation effect is achieved if the chromium content is not less than 10.5%. Other elements (nitrogen, titanium, nickel, molybdenum) enhance corrosion resistance of stainless steels.
A stainless steel heated to 900-1400ºF (482-760ºC) during welding may lose its corrosion resistance because of depletion of chromium consumed for formation of chromium carbides. This effect is called sensitization.
A damage of the passive film may cause intensive localized corrosion (pitting corrosion). Passive oxide layers are dissolved in electrolytes containing sulfates and chlorides.
Phosphate and chromate ions stabilize passive films promoting repair of its defects.
to top
Related internal links


