to Metals
to Corrosion and oxidation
Pourbaix diagrams
Dr. Dmitri Kopeliovich
Pourbaix diagram (Electrode potential / PH diagram) is a graphical presentation of the thermodynamic equilibrium states of a metal-electrolyte system.
Pourbaix diagrams are plotted in the axes Electrode potential of the metal vs. PH of the electrolyte.
Oxidizing conditions are described by the top part of the diagram (high positive electrode potential).
Reducing conditions are described by the bottom part of the diagram (high negative electrode potential).
Acidic solutions are presented in the left side of the diagram (PH lower than 6).
Alkaline solutions are presented in the right side of the diagram (PH higher than 6).
The lines of the diagrams dividing different zones of the equilibrium states are calculated by the Nernst equation:
E = E0 - (0.059/n)*lnCion
Where:
E0 - Standard electrode potential, V;
n - number of electrons transferred;
Cion - molar activity (concentration) of ions.
Pourbaix diagrams allow to determine the corrosion behavior of a metal in water solutions i.e. the direction of electro-chemical processes and the equilibrium state of the metal at a certain electrode potential in a water solution at a certain value of PH.
Normally the Poubaix diagrams are built for the water solutions with the concentrations of metal ions 10-6M and at the temperature 298K (77ºF/25ºC).
An example of a Pourbaix diagram for the system iron-water is presented in the picture.
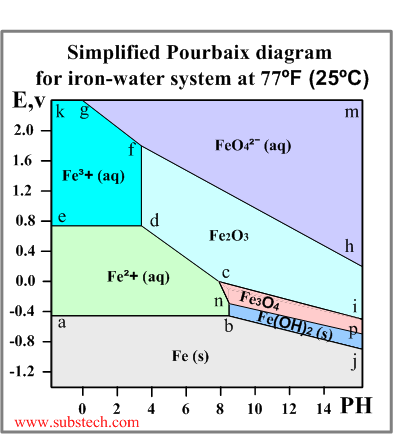 The diagram defines the following zones of the equilibrium states:
The diagram defines the following zones of the equilibrium states:
- below the line a-b-j: Solid iron (immunity zone). The electrochemical reactions in this zone proceed in the direction of reduction of iron ions. No corrosion occurs in this zone.
- a-b-n-c-d-e: Aqueous solution of ion Fe2+ (corrosion zone). Metallic iron oxidizes in this zone.
- e-d-f-g-k: Aqueous solution of ion Fe3+ (corrosion zone). Metallic iron oxidizes (corrodes) in this zone.
- h-f-g-m: Aqueous solution of ion FeO42- (corrosion zone).
- c-d-f-h-i: Solid ferrous oxide Fe2O3 (passivation zone). Iron oxidizes (corrodes) in this zone however the resulted oxide film depresses the oxidation process causing passivation (corrosion protection of the metal due to formation of a film of a solid product of the oxidation reaction).
- n-c-i-p: Solid oxide Fe3O4 (Fe2O3*FeO) (passivation zone). The oxide film causes passivation.
- b-n-p-j: Solid hydroxide (II) Fe(OH)2 / FeO*nH2O / green rust (passivation zone).
Horizontal lines of the Poubaix diagrams correspond to the redox reactions, which are independent of PH.
Vertical lines of the Poubaix diagrams correspond to the non-redox reactions (electrons are not involved), which are dependent on PH.
Diagonal lines of the Poubaix diagrams correspond to the redox reactions, which are dependent on PH.
Here are some of the reactions and the corresponding lines of the Fe-H2O Pourbaix diagram:
- a-b: Fe(s) = Fe2+(aq) + 2e- Redox reaction independent of PH. The equilibrium occurs at the electrode potential value -0.44V, which is equal to the standard electrode potential of iron (see the Electrochemical series).
- e-d: Fe2+(aq) = Fe3+(aq) + e- Redox reaction independent of PH.
- d-f: 2Fe3+(aq) + 3O2- = Fe2O3(s) Non-redox reaction dependent on PH.
- b-n: Fe2+(aq) + 2OH-(aq) = Fe(OH)2(s) Non-redox reaction dependent on PH.
- c-d: 2Fe2+(aq) + 3H2O = Fe2O3(s) + 6H+(aq) +2e- Redox reaction dependent on PH.
- b-j: Fe(s) + 2OH-(aq) = Fe(OH)2(s) + 2e- Redox reaction dependent on PH.
Limitations of Pourbaix diagrams:
- The diagrams provide no information about the kinetic parameters of the corrosion reactions.
- The diagrams consider pure metals and aqueous solutions at standard conditions (temperature 298K, pressure 1 bar, ion concentration 10-6M). Thermodynamic conditions of corrosion for alloys and for non-standard conditions differ from those described by Pourbaix diagrams.
- The diagram do not take into account non-ideal behavior of aqueous solutions.
- Some thermodynamic data used for building diagrams are not precise enough.