Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Coating technologies
Electroplating
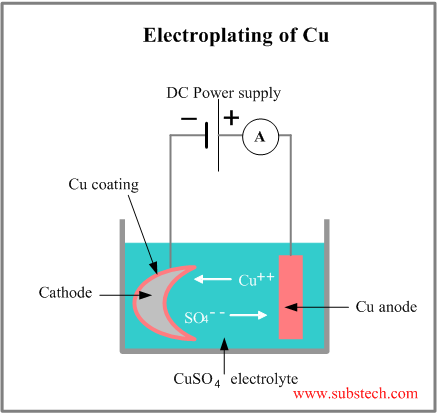
Electroplating is a process of coating deposition on a part, immersed into an electrolyte solution and used as a cathode, when the anode is made of the depositing material, which is dissolved into the solution in form of the metal ions, traveling through the solution and depositing on the cathode surface.
A scheme of the electroplating of copper in the aqueous solution of copper sulfate (CuSO4) is shown in the picture:
Positively charged copper ion moves towards the negative cathode and when it reaches the cathode surface it accepts two electrons, converts to the copper atom and deposits on the cathodes surface.
Faraday’s law
The amount of the deposited material is directly proportional to the amount of electric charge, passed through the circuit.
Since amount of electric charge Q=I*t (I – electric current, t – time), then the Faraday’s law may be expressed by the formula:
W = Itμ/(nF),
Where
W – weight of the deposited material;
μ – weight of one mole of the metal;
n - number of electrons transferred by the ion into solution (n=2 in the above example);
F – Faraday’s constant, F = 96485 Coulombs.
Cathode efficiency is the ratio of the actual amount of the deposited material to the theoretical amount that should be deposited.
Current efficiency is the percentage of current, which is actually used for the deposition at the cathode or for the anode dissolving (not including the current used for the side reactions).
Throwing power – uniformity of the thickness of a coating deposited on irregularly shaped part.
Leveling is ability of electroplating process to deposit smooth uniform coating on the rough surface.
Leveling is achieved by addition of leveling agents into the electrolyte solution.
Brightening is an ability of electroplating process to deposit bright fine-structure coating.
Brightening is achieved by addition of brighteners into the electrolyte solution.
to top
Electroplating methods
- Rack plating
Large and complex parts are coated on racks – copper wire structures, holding the parts.
- Barrel plating
Small parts in a batch are plated in rotating barrels.
Electroless plating is a process in which plating is achieved entirely by chemical reaction occurring at the part surface in the solution with no electric current involved.
In electroless plating the part surface serves as a catalyst for the reaction.
The source of the electrons, required for reducing metal ions, is a reducing agent (reductant).
Nickel, copper, gold can be electroless plated.
Electroforming is a process of making metal articles by electroplating of the metal onto a pattern (mold, mandrel), followed by removal of the deposited layer.
Fine molds for compact disks, screen-printing cylinders, metal bellows and other high accuracy micro parts are manufactured by the electroforming method.
to top
Related internal links
to Metals
to Coating technologies